The largest medical portal dedicated to damage to the human body. Consultation with a doctor.
The article talks about the signs of an alkaline skin burn. First aid measures and treatment methods are described. An alkali burn is a severe chemical injury. Most often it happens at work, but it can also happen at home. It should be noted that household injuries are not so dangerous because highly concentrated solutions are not used.
Chemical burns are one of the most dangerous injuries
- How it happens
- How it manifests itself
- Treatment First aid measures
- Basic treatment
What is the difference between an acid and an alkali?
These two substances are antipodes. The interaction of alkali and acid leads to the formation of water and mutual destruction of each other. In this case, the reaction proceeds quite violently, with hissing and an increase in the temperature of the interaction medium.
Alkali , unlike acid, is more dangerous for human skin . Although many believe that the opposite is true. Alkali quickly forms a severe burn due to deep penetration into the skin, and it is not easy to wash off.
It is possible to determine what substance is in the container even without special equipment. It is enough to purchase a litmus strip. It has a special composition that reacts to acidic and alkaline environments. Litmus dipped into an acid will turn red , and in an alkaline environment it will take on a blue tint.
First aid for burns with acids and alkalis
Skin injuries from alkaline substances are classified as chemical burns and are more dangerous than thermal injuries or even acid burns. If you receive this type of injury, it is important to act correctly before the doctor arrives. First aid for alkali burns has some characteristic differences from measures for thermal injuries.
First aid rules
If you get a burn from alkali, what should you do? They can be at work or at home. In enterprises that use concentrated acidic, alkaline substances or transport them, injuries occur when safety rules are not followed. In everyday life, you can burn yourself with quicklime, ammonia solution, or caustic soda.
Alkali damage is characterized by the continuation of its effect on tissue even after the reagent has ceased contact with the skin. In this regard, first aid is characterized by neutralizing the effect of the alkaline substance. You should proceed according to the algorithm:
- Remove the victim's clothing.
- Rinse the affected area with cool water (keep the wound surface under running water for at least 20 minutes). It is prohibited to remove the reagent from the skin using wet wipes, cloth, or towels. Rubbing the damaged area will cause the alkali (acid) to penetrate even deeper and worsen the damage.
- Treat the wound with citric acid or vinegar dissolved in water.
- Cover with a wound dressing without using ointments.
- Call an ambulance.
Often an alkali burn at home occurs due to careless handling of quicklime. Mostly the hands and eyes are affected. What to do with lesions in such cases? Many would answer “quickly wash off the reagent from the skin, rinse the eyes.” But these actions are the most common mistake that aggravates trauma.
Emergency care if quicklime gets on the skin and mucous membranes does not include rinsing with water. The reagent reacts with it and the effect of the chemical intensifies.
PMP for eye burns with alkali, quicklime (ground or lump) lime should:
- Rinse the organs of vision with a solution of disodium salt of ethylenediaminetetraacetic acid 3% (Na2EDTA). For this purpose, solutions of Versen, Trilon B, Complexon 3, Helaton are used.
- After you have managed to neutralize the alkali, you can rinse your eyes with water.
- Examine the organs of vision under the eyelids for reagent residues and remove them with a clean napkin.
- Apply Floxal or other drops with an antiseptic effect to your eyes. For severe pain, Novocaine drops should be used.
- Place Korneregel regenerating eye ointment behind your eyelids.
- Call an ambulance or take the victim to a medical facility.
Emergency help if quicklime gets on your skin is to remove the reagent using a dry (!) method:
- If necessary, remove clothing.
- Using a dry napkin or cloth, carefully remove the lime from the wound surface.
- Lubricate the injured area with oil, fat or rich cream.
- Apply a sterile bandage.
- Call a doctor.
If alkali or acid gets into the mouth, pharynx or esophagus, a chemical burn can cause serious breathing problems. Signs of damage to the internal tract by chemicals will be: acute pain, vomiting containing mucous particles, salivation, difficulty breathing.
Providing emergency assistance in these cases consists of the following actions:
- if affected by acid, the victim must be given a soda solution to drink;
- if an alkaline substance is ingested, give the patient a drink of an aqueous solution with acetic or citric acid 2%;
- rinse the stomach;
- give milk to drink;
- give 50-100 grams to drink. oils;
- call a doctor.
In what cases does it cause tissue necrosis?
Prolonged contact and high concentration of alkali can cause wet (colliquation) tissue necrosis. This necrosis is worse and more dangerous than dry (coagulative) necrosis from an acid burn.
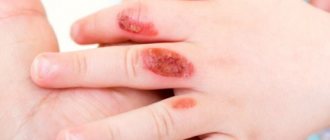
Under the soft, loose scab, there is a weeping wound, swelling, hyperemia, and the release of cloudy exudate. The process of dying spreads deep and diametrically. Infection and suppuration of the wound area often occur.
In such cases, the patient needs surgical treatment, removal of necrotic tissue, and skin grafting.
Treatment methods
An alkali burn is a serious injury that requires proper treatment to prevent the wound from becoming more severe and becoming infected.
In a medical institution, the wound surface is disinfected with solutions of Dioxyzol or Novoimanin. Sintomycin ointment is used to protect the injured area from the development of pathogenic microflora. Oxycyclosole spray is indicated for the treatment of deep burns. It has an antibacterial effect and eliminates allergic manifestations.
For pain relief, the patient is prescribed anesthetic pastes for local use and injections with Lidocaine, Trimecaine, Analgin. For III-IV degree burns, the use of sedatives (Relanium, Valoserdin, Persen) and therapy aimed at detoxification are indicated. Ringer's or Lasix solutions are used intravenously.
For severe burns, treatment cannot be done without antibiotics, and shock and swelling are eliminated with glucocorticosteroid drugs. To accelerate tissue restoration, Retinol, Solcoseryl, Aevit, Aekol are used.
If the mucous membrane of the mouth and pharynx is damaged, gargling with antiseptic solutions (Aqualor, Anestezin, Miramistin) is added.
At home, it is possible to treat burn wounds from exposure to alkali only with mild degree I of damage, sometimes II in the absence of a threat to health. Therapeutic measures include the use of Panthenol, Syntomycin, Levomekol, Bepanten, Sulfargin, Oxycyclosol. Traditional methods are not excluded: egg white, washing the wound with decoctions of St. John's wort and calendula, aloe juice, golden mustache.
What happens if you drink lye?
A person who drinks alkali will experience the following symptoms:
- Mouth burn. Soft tissues swell and turn red. The man is suffering from severe pain. Gradually, swelling turns into ulcers;
- Thirst appears. There is a metallic taste in the mouth for a long time;
- Pain in the chest area. Its intensity depends on the concentration of the alkali drunk;
- Severe vomiting interspersed with blood. This is due to the fact that alkali damages internal blood vessels;
- Risk of asphyxia due to intense vomiting or deformed esophageal tissue;
- State of shock. If the concentration of alkali is high, then the pain symptom leads to a person’s frenzy, he stops thinking rationally and sometimes is not even able to call doctors;
- Some time after the initial symptoms, bloody diarrhea occurs.
Analyzing cases of alkali poisoning, doctors came to the conclusion that the stomach may not be affected at all. After all, when the alkali you drink interacts with the acidic environment of the stomach, the former is neutralized .
But if the concentration of the substance is high enough, then there is a possibility of perforation of the esophagus, that is, the formation of a hole in it.
Even the use of small doses of alkaline solutions is fraught with complications, although at first a person may get away with mild symptoms. Therefore, no self-medication, only prompt consultation with a doctor.
The specialist must also be informed of the name of the substance that caused the shipment. This will help you immediately choose a course of treatment and not waste time on additional examinations.
Victims who have fallen into a state of shock often make a number of dangerous mistakes:
- Induce vomiting . It increases the risk of damage to the esophagus several times. After all, the alkaline liquid from the stomach will again go back along the same path that it got inside;
- They use laxatives . Only a doctor can decide how appropriate a particular detoxification method is;
- Drink a solution of citric acid . This mistake is due to the fact that people have a general understanding of neutralizing an alkali with an acid. But what is relevant for household use will not help in any way with poisoning. An acidic solution can only give additional stress to the poisoned body.
What to do if you are burned by alkali?
The main goal of first aid for an alkaline burn is to remove the chemical from the skin. In case of a burn with alkali, it is necessary to rinse and neutralize. Further treatment and human health depend on how quickly first aid is provided. Therefore, it is important to know how to do this.
Sequence of actions when providing first aid:
- Immediately remove clothing from damaged areas. This will prevent further spread of fluid throughout the body;
- In order to eliminate the influence of the reagent, it is necessary to thoroughly rinse the affected area with running water. The duration of this manipulation should be 20 minutes. If washing is not done immediately, then you need to spend about half an hour;
- Do not lubricate the injury with oil or fat-like ointments;
- You cannot remove the alkaline solution from the skin using a cloth, this will cause the injury to spread over large areas;
- Neutralize alkali using weak acidic reagents. Solutions of vinegar essence or lemon juice are well suited for this. They are always available at home;
- To temporarily reduce pain, apply a bandage soaked in novocaine;
- If you receive a second degree burn, a sterile bandage must be applied. This will prevent the penetration of pathogenic microorganisms into the wound;
- Next, an ambulance is called.
For minor skin damage, alkali can be treated at home. For more dangerous injuries, hospital treatment is required.
Features of a chemical burn
Alkali burns most often lead to dangerous complications. For example, some other aggressive substances immediately react with the skin. It is corroded, but the main part of the chemical does not penetrate deeper.
With alkali everything is different. It may not form a burn crust and quickly penetrates into the deep layers of the skin. At the site of interaction between alkali and skin, a loose, light-colored scab appears. Damaged tissue is released slowly, making healing difficult. This also increases the risk of scarring at the site of alkaline burns.
When a chemical comes into contact with the skin, it appears:
- Irritation;
- Edema;
- Redness;
- Increasing pain that becomes unbearable in case of a burn with high concentration alkali;
- Numbness.
In some cases, victims report feeling as if their skin was “soapy.”
If the concentration of alkali was weak and it did not act on the skin for long, then the person would suffer a superficial burn. The skin will become slightly burgundy, a dull pain and burning sensation will appear.
In severe cases, when deep layers of skin are damaged, pus may form underneath. This creates an additional risk of inflammation.
Treatment
All necessary manipulations and treatment procedures are carried out only after determining the extent of the injury and its location.
To treat a burn with alkali, the following therapy is prescribed:
painkillers to relieve acute pain;- antibacterial therapy to eliminate the risk of infection and development of infection at the site of the lesion;
- treating the affected area on the skin with water-soluble ointments (Levomekol, Levosin);
- When receiving a 4th degree burn, the victim undergoes surgery, during which dead tissue is removed and wound defects are eliminated using plastic surgery.
How to wash off lye?
In everyday life, people often get burns from quicklime. It is just an alkali. Many victims in this case instinctively try to wash off the reagent with plain water. Not only will this not help, but it will further enhance the effect of the alkali.
If quicklime gets on your skin, remove it with a dry cloth. Then the damaged skin is lubricated with vegetable oil. If the inflammation is severe and causes acute pain, then you should consult a doctor.
Other types of alkalis should be washed off with cool water . To effectively get rid of the reagent, you need to keep the damaged skin under the stream for about 15 minutes.
Do not dry your skin with a towel. Loose scabs may have time to form on the skin, so friction causes the skin to become even more injured.
Prognosis and prevention
The prognosis of an injury depends on its severity and duration of exposure to alkali. Experts determine it by the lesion severity index. 1% of the affected superficial burn is equal to 1 unit.
A prognosis of less than 30 units is considered favorable. up to 60 units Unfavorable 91 or more units. Severe degrees leave scars on the skin, damage muscles and can lead to disability of the victim, and in the absence of timely treatment, to death.
The best prevention of chemical burns is considered to be compliance with safety precautions at work and at home. When working with alkalis, it is necessary to use protective suits, wear gloves, and protect your face with a mask and goggles.
Alkali burns are injuries that are quite difficult to treat. Timely first aid and competently conducted therapy not only helps to quickly restore the victim’s health, but in some cases saves his life.
How to neutralize alkali on the skin?
The peculiarity of alkali is that the alkali can still affect the skin for some time, even if there are no longer traces of the substance itself. Therefore, when the affected area of skin is washed with water, it should be treated with a vinegar solution . This acid perfectly neutralizes alkali residues.
If the burn is small, then after washing with water and vinegar, you can independently use various medications to speed up healing. An inexpensive Levomekol ointment is suitable, which has an antimicrobial effect and eliminates inflammation.
If a festering wound appears, then Levosin will help. The ointment also enhances skin regeneration processes.
It is important to note that the use of drugs is relevant only in the absence of complications . Skin that does not heal for a long time after an alkaline burn or has acquired an unnatural color is a signal for alertness and qualified treatment.
It turns out that getting alkali on the skin can cause a long-healing burn with complications. Simply washing the wound and treating it is not enough. Especially if the concentration of the substance was high or a significant area of skin was damaged. In this case, you cannot do without the help of a doctor.
ALKALI
ALKALI
- a general name for strong water-soluble bases. In medicine, alkalis are used as irritants, cauterizers, disinfectants and antiseptics. Calcium hydroxide - slaked lime (see Lime) in the form of aqueous solutions (lime water, Aqua calcarea) is used to neutralize acids.
Alkalies primarily include alkali metal hydroxides (see) NaOH, KOH, usually called caustic alkalis (see Caustic soda, Caustic potassium). Alkaline earth metal hydroxides (see) - Ca(OH)2, Ba(OH)2, as well as ammonium hydroxide NH4OH (see Ammonia), aqueous solutions of aliphatic amines (see) and hydrazine (see), also have properties of alkalis. tetraalkylammonium hydroxides. Alkaline salts of weak acids have a chemical effect similar to alkalis (see Acids and bases) - carbonates (soda, potash), tetraborates (for example, Na2B407), trisubstituted phosphates (for example, Na3P04), etc. Aqueous solutions of alkaline alcoholates exhibit the properties of caustic alkalis metals (see Alcohols). In aqueous solutions, caustic alkalis almost completely dissociate (see Dissociation in chemistry) into alkali metal and hydroxyl ions (OH~), due to which they are the strongest bases; this also determines the high basicity of tetraalkylammonium hydroxides, which is not inferior to caustic alkalis. The lower basicity of alkaline earth hydroxides and ammonium hydroxide NH4OH is associated with a lower degree of their dissociation. The alkaline properties of salts of weak acids are explained by their hydrolysis with the formation of caustic alkalis: Na2C03 + H20 NaHCOo-f-+ NaOH.
Alkalis - hydroxides of alkali and alkaline earth metals - are colorless crystalline substances, they are hygroscopic, especially caustic alkalis, which diffuse in air. All alkalis vigorously absorb carbon dioxide (carbon dioxide C02) from the air, turning into carbonates, so they are stored hermetically sealed. Caustic alkalis (except for lithium hydroxide LiOH) are highly soluble in water (up to 50% and higher), as well as in methyl and ethyl alcohol. Alkaline earth metal hydroxides are moderately soluble in water, their solubility increases from Ca(OH)2 to Ba(OH)2. The dissolution of alkali in water occurs with a significant release of heat due to the formation of hydrates (see Hydration). Alkali solutions have pH values (see pH value) equal to approximately 11 - 14, and sharply change the color of acid-base indicators (see). When interacting with acids, alkalis form salts (see), which is widely used in practice not only for neutralizing acids, but also as an analytical method (see Neutralization method). When fats (see) are treated with alkali, they are saponified (see), resulting in the formation of salts of fatty acids - soaps (see). Caustic alkalis are highly chemically active and react with many substances and materials. Some metals - aluminum, zinc, tin, etc. - react with solutions of caustic alkalis, for example, aluminum is corroded even by weak alkalis (soda, ammonia). In concentrated solutions and especially in the molten state, caustic alkalis destroy glass, porcelain and even platinum (in the presence of oxidizing agents, including air), which should be taken into account when working with molten alkalis (in these cases, utensils made of iron, nickel or silver are used). Caustic alkalis corrode and destroy animal and plant tissues due to their hydrolytic effect on proteins, but plant tissues are subject to such alkali action to a lesser extent.
The use of alkalis in disinfection is based on their properties to hydrolyze proteins, saponify fats, break down carbohydrates, and destroy microbial cells (see Disinfection, Disinfectants).
Of the alkalis, the most active is caustic soda, or caustic soda (NaOH), which has a bactericidal, virucidal, and in high concentrations at elevated temperatures, a sporicidal effect; its 2-4% solutions are used for intestinal and droplet infections of bacterial and viral etiology, and a 10% solution heated to 75° is used for anthrax. These solutions are used to disinfect the premises of public catering establishments, sheepskin and fur factories, storage facilities for raw materials of animal origin, as well as premises for animals.
Sodium metasilicate (a mixture of Na20 and Si02) has bactericidal, bleaching and detergent properties; It is used to a limited extent for disinfection of intestinal and droplet infections of bacterial etiology, since a 2% solution of sodium metasilicate spoils paint, rubber gloves, discolors fabrics, and leaves indelible stains on glass. Sodium carbonate (soda) has a weak bactericidal effect, its 1 - 2% solutions are used as an auxiliary agent when boiling to disinfect dishes, linen, patient care items, as well as for wet-mechanical cleaning of premises, cleaning equipment, furniture.
Ammonium hydroxide NH4OII (see Ammonia) has weak bactericidal properties and is used mainly to neutralize formaldehyde after treating various objects with it.
Quicklime CaO is used for disinfecting surfaces by whitewashing them at the rate of 1 liter of 10% aqueous solution (lime milk) per 1 l2, as well as for treating excretions and soil.
Alkalis as an occupational hazard. Alkalies are used in the textile, paper, leather industries, soap making, etc. They can enter the human body through inhaled air or through the mouth (by accidental or intentional ingestion). Alkalies have a sharp irritating and cauterizing effect on the skin and mucous membranes. Caustic alkalis are deeper than acids (see Sulfuric acid, Hydrochloric acid), penetrate tissues, dehydrate them, saponify fats, and form alkaline albuminates with proteins, causing liquefaction necrosis. The severity of the damage depends on the concentration and temperature of the alkali solution and its pH value. When contact with the skin, alkalis cause burns (see), the healing of which takes longer than in the case of exposure to acids, since deeper tissues are involved in the process. The general toxic effect of alkali is weakly expressed and manifests itself mainly in cases of chronic poisoning.
With constant contact of alkali with the skin, the development of nodular dermatitis (see), the appearance of painful, long-term non-healing ulcers (such as “bird's eyes”), and eczema (see). The skin becomes dry, cracks form between the fingers, nails become thinner, break, become deformed, and separate from the nail bed. Stopping contact with alkali leads to rapid recovery.
With chronic exposure to dust or alkali aerosol on the respiratory tract, chronic rhinitis (see), pharyngitis (see), bronchitis (see), pulmonary emphysema (see) can develop. As a result of ingestion of dust or aerosol particles, gastritis occurs (see); in some cases, ulcerative lesions of the gastric mucosa can be observed.
In case of acute poisoning by aerosols or alkali dust in high concentrations that enter the respiratory tract with inhaled air, severe irritation of the mucous membrane of the upper respiratory tract occurs and tracheobronchitis develops. In acute oral alkali poisoning, a burn of the oral cavity, esophagus, stomach occurs, painful vomiting, diarrhea with blood, severe thirst, urinary retention, and later toxic nephrosis, pneumonia, purulent mediastinitis, pleural empyema, heart failure up to collapse and shock develop. Sometimes laryngospasm, swelling of the larynx and lungs, and psychomotor agitation are noted (especially in case of ammonium hydroxide poisoning). Perforation of the stomach wall often occurs, sometimes esophageal-gastric bleeding that is difficult to stop, peritonitis and (without timely prevention) the formation of stenoses of the esophagus, cardiac and pyloric parts of the stomach more extensive than with acid poisoning are noted.
When alkali gets into the eyes, acute irritation of the conjunctiva, clouding and ulceration of the cornea, damage to the deeper layers of the eye - the iris, vitreous body and retina - occurs, which can result in blindness.
First aid and emergency treatment when alkali gets on the skin consists of immediately washing off the alkali with a stream of water for 5-10 minutes, after which lotions from a 5% solution of citric, tartaric or acetic acids are applied to the affected area. If alkali gets into the eyes, they are immediately washed with a copious stream of water for 10-30 minutes; if calcium hydroxide gets into the eyes, the eyes are washed with a 5% solution of ammonium chloride or a 0.01% solution of calcium thetacine, after which a 0.5% solution of dicaine is instilled or 1% novocaine solution.
In case of acute inhalation poisoning with alkali, the nasopharynx is washed with water with citric acid diluted in it, the victims are allowed to breathe oxygen with acidified water vapor, codeine is prescribed orally, and, if indicated, cardiac medications. In case of oral alkali poisoning, it is necessary to carry out the entire complex of emergency measures used in case of poisoning (see), that is, lavage of the stomach with cold acidified water through a tube lubricated with vegetable oil; before rinsing, 1 ml of a 1% solution of morphine and a 0.1% solution are injected subcutaneously atropine. Measures are taken to treat burn shock (see) - polyglucin, glucosonic acid mixture intravenously; 2 ml of 2% papaverine solution, 1 ml of 0.2% platifidlin, 1 ml of 0.1% atropine solution are injected subcutaneously (up to 6-8 times a day). With the development of bleeding - local hypothermia of the stomach. The victim must be provided with peace and warmth; in severe cases, urgent hospitalization is required. Further treatment, as with chronic HC intoxication, is symptomatic.
Work ability examination. Issues of examination of ability to work, medical and labor rehabilitation for alkali intoxication are resolved taking into account the severity of clinical symptoms of intoxication and hygienic characteristics of working conditions.
Preventive measures when working with alkali include sealing the loading and unloading of the GC, continuity of the technological process; the equipment must be equipped with shelters with local exhaust ventilation (see). Dust-producing processes (crushing, transportation, etc.), as well as work with hot alkali solutions, should be under special control. Workers must be provided with safety glasses (see), respirators (see), special clothing (see Special clothing) and gloves. Medical examinations are carried out periodically (see Medical examination).
The maximum permissible concentration in the air of the working area is set for caustic alkalis (in terms of caustic soda) 0.5 mg/m3, for calcium carbonate (limestone) - 6 mg/m3, sodium carbonate (soda ash) - 2 mg/m3.
Alkalis in forensic medicine. Alkali poisoning is relatively rare and occurs mainly when ammonium hydroxide, caustic soda (caustic soda), caustic potassium, slaked [Ca(OH)2] and quicklime (CaO) are ingested orally into the body as a result of an accident or for suicidal purposes. The lethal dose for alkali poisoning is 10-20 g, and the mortality rate reaches 50%. Death can occur within the next few hours after alkali enters the body from exotoxic shock or toxicogenic collapse. acute respiratory failure (due to inhibition of the function of the respiratory center, laryngospasm, toxic pulmonary edema), and later - from cardiovascular failure or complications: pneumonia, purulent mediastinitis, pleural empyema, toxic nephrosis, etc. In forensic cases examination of a corpse notes characteristic deep loose (colliquation) necrosis of the stomach wall (especially in case of caustic potassium poisoning) without a clear boundary with intact tissue. The wall of the stomach is soapy to the touch, covered with a gray or greenish-brown scab (due to the formation of alkaline hematin color. Edema of the mucous membrane of the larynx, pharynx, inflammatory changes in the mucous membrane of the trachea and bronchi, pulmonary edema and emphysema, swelling of the substance and congestion of the blood vessels of the brain are also noted , plethora of internal organs, pinpoint hemorrhages iodine serous membranes and in tissues. Reveal edema, plethora, hemorrhages and necrosis of the mucous membrane and sublytic layer of the esophagus and stomach, infiltration of their walls with leukocytes, protein degeneration of parenchymal organs with edema of the interstitial tissue, thrombosis of small vessels.
During forensic chemical testing, alkalis are isolated from biological material by dialysis. For an alkaline reaction, an alcoholic solution of phenolphthalein and excess barium chloride are added to the dialysate. If the dialysate remains pink in color, it indicates the presence of alkali. The chemical nature of the cation (Na+, K+, Ca2+) is determined by the formation of a characteristic crystalline precipitate when sodium pyroantimony acid, tartaric acid and ammonium oxalate are added to the dialysate; NH ions are determined by the blueness of red litmus paper with a negative test for hydrogen sulfide. The quantitative determination of alkali is based on the titration of an aqueous extract from the tissue being tested with an acid in the presence of an indicator (see Titrimetric analysis).
The task of a forensic medical examination when examining victims may include establishing the severity of bodily injuries caused by the action of alkali, and when examining corpses - establishing alkali poisoning as the cause of death. The assessment of the severity of bodily injuries in these cases is carried out in the usual manner (see Injuries in forensic medicine); Diagnosis of alkali poisoning as the cause of death is made based on an assessment of anamnestic and clinical data, autopsy results, chemical and histological studies.
Bibliography: Avdeev M.I. Forensic medical examination of a corpse, p. 367, M., 1976; V and shko in V. I. Guide to disinfection, disinsection and deratization, p. 96, M., 1956; aka, Antimicrobial agents and methods of disinfection for infectious diseases, p. 33, M., 1977; Harmful substances in industry, ed. N.V. Lazarev and I.D. Gadaskina, vol. 3, p. 320, L., 1977; 3 a g o r a E. Industrial ophthalmology, trans. from Polish, p. 111 and others, M., 1961; Nekrasov B.V. Fundamentals of general chemistry, vol. 2, p. 213 and others, M., 1973; Guide to forensic medical examination of poisonings, ed. R.V. Berezhny et al., p. 79, M., 1980; Forensic Medicine, ed. A. R. Denkovsky and A. A. Matyshev, p. 229, L., 1976. A. I. Tochilkin; I. V. Buromsky (court), E. N. Marchenko (gig.), N. F. Sokolova (epid.).
Possible complications
Complications of alkali burns can be general and local. General ones develop in case of extensive and deep damage:
- Burn hypovolemic shock due to fluid loss;
- Poor circulation due to blood thickening, thrombosis;
- Impaired liver and kidney function associated with intoxication;
- Sepsis due to infection of extensive burn surfaces.
Local consequences can be in the form of wound suppuration, prolonged non-healing, the formation of rough keloid scars that limit mobility or are a cosmetic defect.
On the part of the eyes, the most common development is clouding of the cornea - a cataract; with severe burns, the deeper media of the eye are also affected, up to complete loss of vision.
Treatment of burn wounds at home
If the burn wounds are small and not very deep, then treatment of the burn with alkali can be carried out at home using medications prescribed by a doctor, supplementing them with folk remedies, if possible. You need to visit your doctor regularly and follow his recommendations.
Use of drugs
Alkaline burns are dangerous because infection easily develops in areas of wet necrosis. Therefore, in the acute stage, antibacterial agents are prescribed - antiseptic solutions. They moisten a sterile napkin and change it 2-3 times a day until the wound is completely cleansed and “dried” so that there is no purulent discharge. Solutions of Miramistin and Chlorhexidine work well.
To quickly remove necrotic tissue, Iruksol enzyme ointment is widely used; it dissolves dead areas and has an antiseptic effect. First, the wound is washed with an antiseptic, then ointment is applied for a day.
A cleansed granulating wound needs agents that stimulate the healing process. The most effective ointments are Solcoseryl, Actovegin, and if there is still a lot of discharge, use a Peloidin solution prepared on the basis of medicinal mud. Wound dressings are done once a day.
Traditional methods
Among the folk methods for burns, the application of all kinds of gruels and mixtures of grated vegetables, fats and oils should be excluded. Preference should be given to products that undergo heat treatment. You can treat alkali burns with decoctions and herbal infusions:
- Chamomiles;
- Calendula;
- Mint;
- Hop cones;
- St. John's wort.
The possibility of using any folk medicine for burns must be agreed with a doctor.
You can prepare an herbal mixture by taking equal parts of each component, brew 1 tablespoon of the mixture with a glass of boiling water, it is better to hold it in a water bath for 10-15 minutes, then strain into a boiled bowl and cool. Apply to the wound on a moistened sterile napkin 2-3 times a day.
Freshly squeezed juice of aloe or Kalanchoe leaves gives a good antibacterial and wound-healing effect.
It is carefully squeezed into a clean container, moistened with a sterile napkin, and applied to the wound daily. A decoction of oak bark and strong tea brewing will help relieve pain and itching around the wound. Prepare at the rate of 1 tablespoon per 1 glass of boiling water, leave on low heat for 5 minutes. Apply several times a day for half an hour.
Pathogenesis
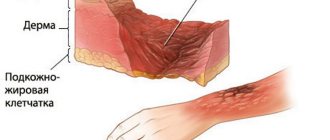
From a chemical point of view, the causes of alkali burns are that upon direct contact and physical-chemical interaction of alkalis (alkali metal hydroxides Na, Ca, K) with human skin, a corrosion-type reaction begins, that is, the aggressive substance corrodes the tissue.
The pathogenesis of a chemical burn with alkali is associated with the fact that an irreversible reaction of alkaline hydrolysis occurs, during which hydroxyl anions of alkali (OH - ) break down the lipids of ceramides and keratins of the stratum corneum of the skin, break the amide bonds of protein molecules of the epidermis and subcutaneous tissue, and cause absorption of interstitial fluid. Complete denaturation of proteins during an alkali burn is completed when the bases bind serum proteins to albumin, resulting in: the osmotic pressure in the cells is disrupted, jelly-like hydrolysis products (albuminates) are formed, damaged skin and soft tissue cells swell and quickly die.
Albuminates can dissolve, but cannot coagulate, so alkali burns are quite deep - with specific wet (colliquation) necrosis. The scab that forms at the burn site has a loose structure, which greatly increases the risk of infection of the burn wound. According to combustiologists, alkali burns are very dangerous and heal more slowly than other chemical burns.
[19], [20], [21], [22], [23], [24], [25], [26]
When should you see a doctor?
It should be taken as a rule that in case of a burn with alkali, regardless of its area and location, it is necessary to consult a doctor. The degree of the burn is difficult to immediately determine, especially for a person without medical education.
In case of burns to any area of the body, the victim must be taken to a trauma center, or the emergency department of traumatology or surgery.
For minor burns, you can see a surgeon or traumatologist at the clinic. Eye burns should be examined by an ophthalmologist as soon as possible. If the injury occurred in a rural area where there is no hospital nearby, you must contact a paramedic-midwife station. The paramedic will provide first aid and decide on further actions.
Features and degrees
A chemical burn with alkali is a serious and dangerous injury due to the nature of the reagent’s action when it comes into contact with the skin. Caustic solutions penetrate deep into the tissues, convert proteins into alkaline albuminates, which is accompanied by the formation of loose, soft whitish scabs. Such wounds take a long time to heal, and over time, darkened crusts form bleeding ulcers. Traces in the form of scars remain at the site of damage.
Depending on the concentration of the reagent, its volume, time of exposure to the skin, tissue, depth and extent of injury, 4 degrees of damage are distinguished:
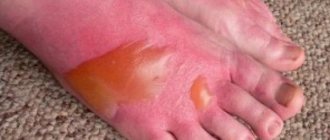
I – only the epidermis is damaged. A minor injury is accompanied by slight swelling, redness of the skin, and burning.
II – the lesion affects the dermis. In the damaged area, blisters with exudate inside are observed. Symptoms of injury are pain, burning, redness, swelling.
III – refers to severe injury. The lower skin layers and subcutaneous tissue are affected. Bubbles of various sizes form; when they burst, they expose the inflamed papillary layer of the dermis. The injury is accompanied by severe pain, swelling, tissue necrosis, and scab formation.
IV is the most severe degree, in which soft tissues, muscles, and ligaments are affected. The lesion can reach the bones. The injury poses a great danger to the health of the victim; surgical treatment and plastic surgery are often required.
ICD 10 code T20-T32.